Abstract
BACKGROUND: Neuroblastoma (NB) is one of the most common childhood malignancies and half of the patients are diagnosed with high-risk disease. Current multimodal treatment regimens only achieve long-term survival in 50-60% of the high-risk patients. In the past two decades, several new treatment strategies, including immunotherapy directed against the NB tumor-associated antigen GD2, have significantly improved the outcome for NB patients. However, many patients still suffer relapsed and refractory disease. The chimeric antigen receptor-modified T cells (CAR-Ts) have shown promising anti-tumor effects in B-cell malignancies. In an earlier trial using 4SCAR-GD2 T cells (NCT02992210), we evaluated 34 refractory/relapsed (R/R) NB patients for safety and long-term survival with a median follow-up of 27.5 months (ranged 2 to 53). We found low to no CAR-T-related toxicities (CRS grade 0-2), and no grade 3 or above CRS. The overall survival (OS) and progression-free survival (PFS) were 48.7±8.7% and 35.3±8.2%, respectively, at 3 years of follow-up. The 34 patients were divided into 2 groups according to their disease status at the time of CAR-T infusion: active disease (n=9) and stable disease (n=25). The PFS (p=0.006) and OS (p=0.017) were significantly worse among patients with active disease upon CAR-T therapy, indicating that patients with active disease did not benefit from the 4SCAR-GD2 therapy. This prompted a change of strategy in the patient enrollment criteria to improve clinical outcomes. In a revised 4SCAR2.0-GD2 study, we enrolled R/R NB patients with stable disease to receive sequential 4SCAR-GD2 T cell infusions and evaluated the anti-tumor effects and in vivo CAR-T persistency.
METHODS: This report included 19 R/R NB patients, with stable disease and without high tumor burden or central nervous system involvement, who were enrolled in the 4SCAR2.0-GD2 study. If disease was stable and the CAR-T level in the blood was reduced below 0.5%, a booster GD2 CAR-T infusion was considered. Autologous T cells were collected and transduced with a safety-engineered lentiviral CAR (4SCAR) carrying the following intracellular signaling domains: CD28-CD27-CD3ζ-iCasp9. The patients received cyclophosphamide and fludarabine conditioning before the first infusion of an average of 2.93x106 CAR-T cells/kg. The toxicity was determined based on the NCI common toxicity criteria. The International NB Response Criteria were used to define response after CAR-T treatment.
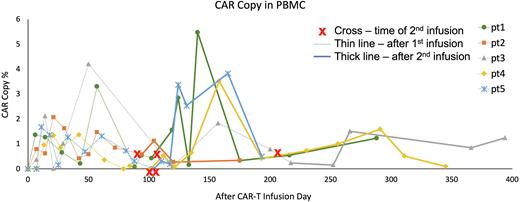
RESULTS: Total 19 patients who had refractory minimal residual disease in the bone marrow were included for evaluation. Besides bone marrow involvement, some had metastatic lesions in the bone or lymph nodes. After the first 4SCAR-GD2 infusion, 12 patients achieved CR in the bone marrow, and 7 did not achieve disease remission. After the CAR-T infusion, the levels of CAR-Ts in the peripheral blood were monitored by qPCR. Once the CAR-Ts were diminished, a booster CAR-T infusion was given. A rise in the CAR-T levels in the blood after the booster infusion is routinely observed; representative CAR-T kinetic graphs of five patients are shown (Figure). Total 10 of the 19 patients received a booster 4SCAR-GD2 infusion. After the booster infusion, seven of the ten patients remained stable, and three had disease progression. The median observation time after the initial CAR-T infusion is 13 months (ranged 7 to 22). The adverse effects observed after the 1st or the 2nd CAR-T infusions were grade 0, 1 or 2 CRS, thrombocytopenia, and localized pain in the peripheral nerve or tumor lesions.
CONCLUSIONS: These results have demonstrated safety and anti-tumor effects of the new 4SCAR2.0-GD2 regimen in R/R NB patients with bone marrow disease. Expanded study is ongoing to address long-term clinical outcomes.
Disclosures
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal